Graphene batteries offer significantly higher energy density, faster charging times, and longer lifespans compared to traditional lead-acid batteries, making them a cutting-edge choice for electric vehicles and portable electronics. Lead-acid batteries, while cost-effective and widely used, suffer from lower energy efficiency and shorter cycle life, limiting their performance in high-demand applications. Explore the advantages and future potential of graphene batteries to understand why they are rapidly transforming energy storage technology.
Why it is important
Understanding the difference between graphene batteries and lead-acid batteries is crucial for selecting energy storage solutions with higher efficiency, longer lifespan, and faster charging capabilities. Graphene batteries offer superior conductivity and lightweight design compared to the heavier, less efficient lead-acid batteries commonly used in traditional applications. Knowledge of these distinctions allows consumers and industries to optimize performance, reduce maintenance costs, and promote sustainable technology adoption. Choosing the right battery technology impacts overall energy consumption, environmental footprint, and device reliability in sectors such as electric vehicles and renewable energy systems.
Comparison Table
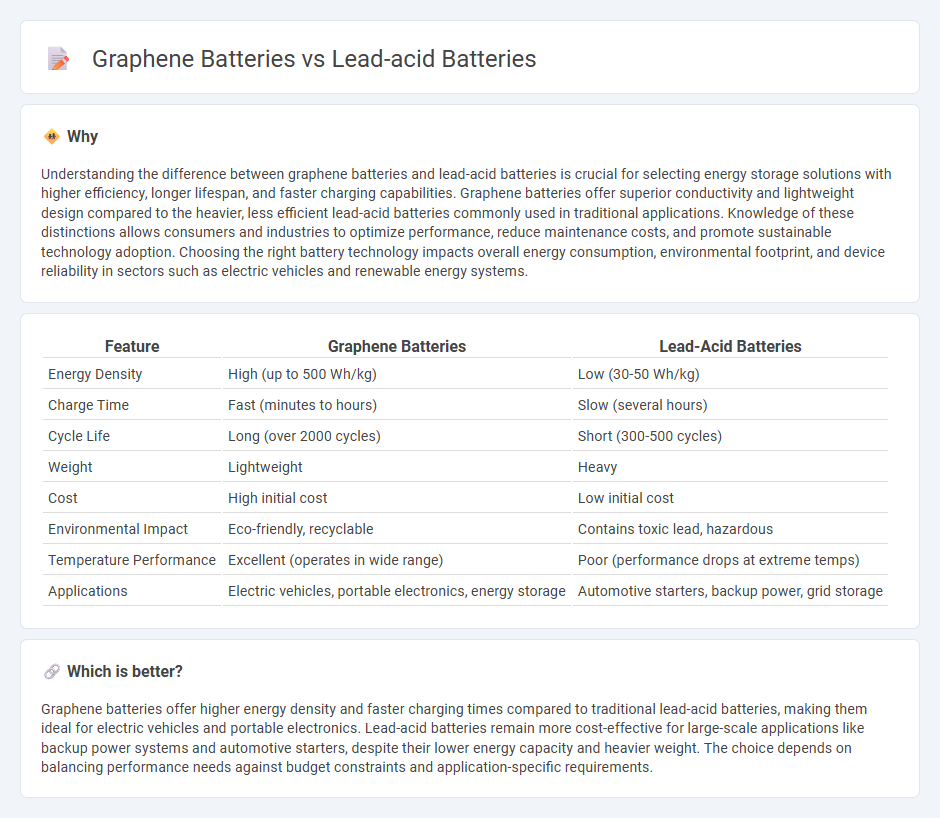
| Feature | Graphene Batteries | Lead-Acid Batteries |
|---|---|---|
| Energy Density | High (up to 500 Wh/kg) | Low (30-50 Wh/kg) |
| Charge Time | Fast (minutes to hours) | Slow (several hours) |
| Cycle Life | Long (over 2000 cycles) | Short (300-500 cycles) |
| Weight | Lightweight | Heavy |
| Cost | High initial cost | Low initial cost |
| Environmental Impact | Eco-friendly, recyclable | Contains toxic lead, hazardous |
| Temperature Performance | Excellent (operates in wide range) | Poor (performance drops at extreme temps) |
| Applications | Electric vehicles, portable electronics, energy storage | Automotive starters, backup power, grid storage |
Which is better?
Graphene batteries offer higher energy density and faster charging times compared to traditional lead-acid batteries, making them ideal for electric vehicles and portable electronics. Lead-acid batteries remain more cost-effective for large-scale applications like backup power systems and automotive starters, despite their lower energy capacity and heavier weight. The choice depends on balancing performance needs against budget constraints and application-specific requirements.
Connection
Graphene batteries and lead-acid batteries are connected through their application in energy storage and power delivery systems, with graphene technology enhancing traditional lead-acid battery performance by improving conductivity and charge capacity. The integration of graphene materials into lead-acid batteries results in faster charging times, increased lifespan, and better thermal stability compared to conventional lead-acid counterparts. This synergy between graphene and lead-acid battery technology is driving advancements in automotive, renewable energy storage, and portable electronics industries.
Key Terms
Energy density
Graphene batteries demonstrate significantly higher energy density compared to traditional lead-acid batteries, offering up to 5 times more energy storage per unit weight. This improvement translates into longer-lasting power for electric vehicles and portable electronics, enhancing overall efficiency and reducing recharge frequency. Explore the advancements in graphene battery technology to understand its potential impact on future energy storage solutions.
Charge cycle lifespan
Lead-acid batteries typically offer 500 to 1,000 charge cycles, while graphene batteries can achieve up to 2,000 to 5,000 cycles due to enhanced conductivity and structural integrity. This extended lifespan translates to longer usage periods and reduced replacement frequency, benefiting applications such as electric vehicles and energy storage systems. Explore the advancements in graphene battery technology to understand its potential impact on energy solutions.
Charging speed
Lead-acid batteries typically charge slower, requiring several hours to reach full capacity due to their chemical composition and internal resistance. Graphene batteries offer significantly faster charging speeds, often completing in minutes, enabled by graphene's superior electrical conductivity and enhanced ion transfer. Explore more about how graphene technology revolutionizes battery performance and charging efficiency.
Source and External Links
Lead-acid battery - The first type of rechargeable battery, invented by Gaston Plante in 1859, widely used in motor vehicles for their ability to provide high surge currents despite having low energy density.
BU-201: How does the Lead Acid Battery Work? - Provides details on how lead acid batteries function, their moderate lifespan, and their advantages in cold temperatures and cost-effectiveness.
About Lead Batteries - Highlights the recycling of lead batteries and the process of manufacturing them, emphasizing their role in various applications.
 dowidth.com
dowidth.com